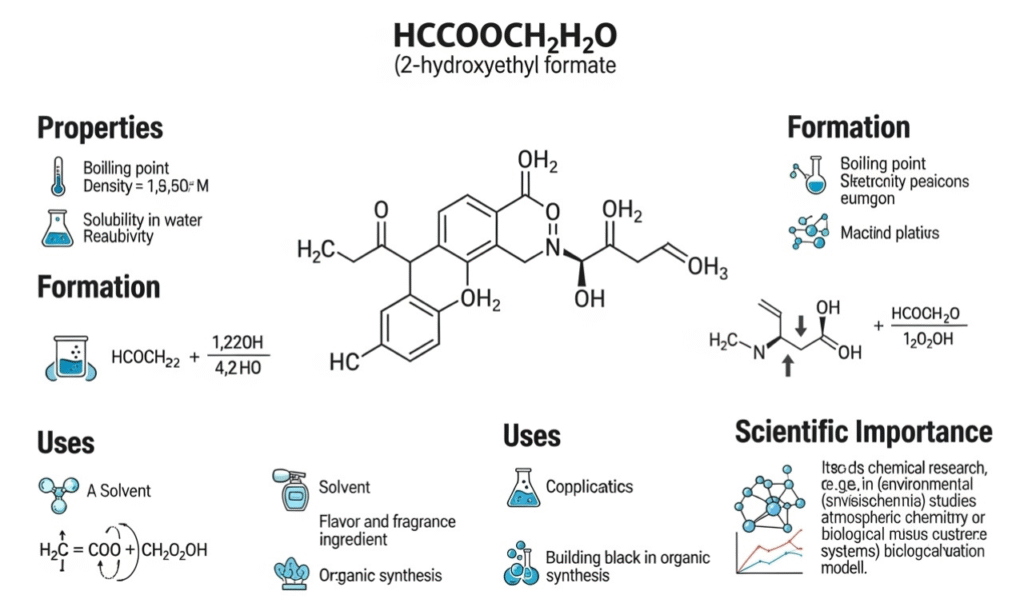
The chemical expression hcooch ch2 h2o may seem unfamiliar at first glance, but it represents a concept used in discussions of organic chemistry, reaction pathways, and structural analysis. In this article, we explore the meaning, behavior, and scientific relevance, along with its applications in education, laboratories, and industrial scenarios. By understanding the individual components and their interactions, learners and professionals can develop clearer insight into how such formulations fit into broader chemical systems.
What Does hcooch ch2 h2o Mean?
To begin, hcooch-ch2 h2o refers to a combined representation of different chemical fragments. While not a standard molecular formula, the expression helps students and researchers analyze how carbon-based groups, hydrogen bonding, and water molecules appear in various reactions. Each part of hcooch-ch2 h2o can be associated with a functional group or component commonly found in organic compounds. This makes it useful as a reference point when discussing hydrolysis, condensation, and various forms of organic synthesis.
Breaking Down the Components
1. The HCOOCH Fragment
The first component, often written as HCOOCH, resembles a portion of an ester or formate structure. Esters are important in organic chemistry because of their pleasant odors, reactivity, and role in biological processes. In the context of hcooch-ch2 h2o, this fragment helps illustrate how ester-like groups can react with water under certain conditions.
2. The CH2 Segment
The central CH2 unit represents a methylene group. Methylene bridges appear in thousands of organic compounds, from simple hydrocarbons to complex biomolecules. When included in the hcooch ch2-h2o expression, it shows how carbon chains can extend, connect, or modify the behavior of adjacent functional groups.
3. The H2O Component
The H2O part signifies the presence of water. Water acts as a reactant, product, or medium in countless chemical reactions. In hcooch-ch2 h2o, the H2O portion suggests the involvement of hydration, hydrolysis, or solvation processes.
How Relates to Chemical Reactions
Hydrolysis Reactions
Hydrolysis is one of the most relevant concepts when studying hcooch-ch2 h2o. Ester-like structures tend to break down in the presence of water, producing alcohols, acids, or intermediates depending on reaction conditions. By using hcooch-ch2 h2o as a teaching example, educators can demonstrate how functional groups behave when attacked by water molecules.
Condensation Reactions
Condensation reactions involve two molecules joining together with the loss of water. The presence of H2O in hcooch ch2-h2o helps explain how water is either produced or consumed during the formation of organic compounds. This is especially useful when discussing polymerization and esterification.
Solvation and Stabilization
Water serves as an excellent solvent for many polar or partially polar molecules. The hcooch-ch2 h2o combination highlights how solvation stabilizes structures, allowing reactions to proceed efficiently in aqueous environments.
Structural Interpretation
Understand Molecular Geometry
Molecular geometry determines how substances interact. When analyzing hcooch-ch2 h2o, students can visualize how carbon, hydrogen, and oxygen atoms align. Understanding bond angles, electron distribution, and spatial arrangement helps predict reactivity.
Functional Group Behavior
Each component behaves according to the rules of organic chemistry. Ester-like fragments undergo nucleophilic attack, methylene groups participate in chain stabilization, and water molecules act as reactive agents. This makes the formula useful as an educational illustration.
Applications in Learning and Research
Educational Uses
Chemistry instructors often use combined formulas like to test students’ knowledge of functional groups and reaction mechanisms. It helps learners break complex structures into understandable parts.
Laboratory Applications
In laboratory contexts, interpreting formulas like, allows researchers to discuss hypothetical pathways, plan syntheses, or identify unknown compounds. While the expression may not represent a single standardized molecule, it still holds relevance when analyzing how organic pieces fit together.
Industrial Relevance
Industries that work with polymers, plastics, pharmaceuticals, and solvents often rely on structures similar to parts of hcoochch2 h2o. Understanding these fragments allows chemists to design safer, more efficient, and more predictable reactions.
Why Learning About hcooch ch2 h2o Matters
Improved Understanding of Organic Chemistry
Using hcoochch2 h2o as a conceptual tool enhances understanding of detailed mechanisms, showing how molecules react in the presence of water or chain-building carbon units.
Better Problem-Solving Skills
Students who work with structures like hcoochch2 h2o become better at interpreting formulas, identifying intermolecular forces, and predicting reaction outcomes.
Foundation for Advanced Chemistry
Knowledge gained from studying hcoochch2 h2o prepares learners for advanced topics such as biochemistry, pharmaceutical chemistry, and environmental chemistry.
Conclusion:
Although hcoochch2 h2o does not represent a single official chemical compound, it serves as an effective conceptual representation for exploring organic structures, reaction pathways, and the role of water in chemical processes. By analyzing its components—an ester-like fragment, a methylene group, and water—students and professionals can deepen their understanding of molecular behavior. The expression highlights the importance of functional groups, solvation, hydrolysis, and condensation reactions in organic chemistry. Ultimately, hcooch stands as a versatile educational example that builds stronger foundations for scientific learning and laboratory practice.